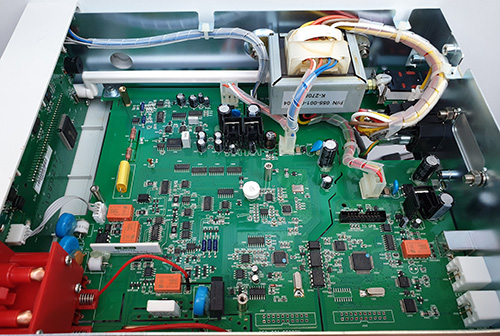
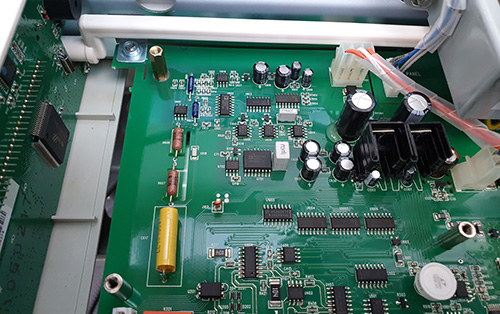
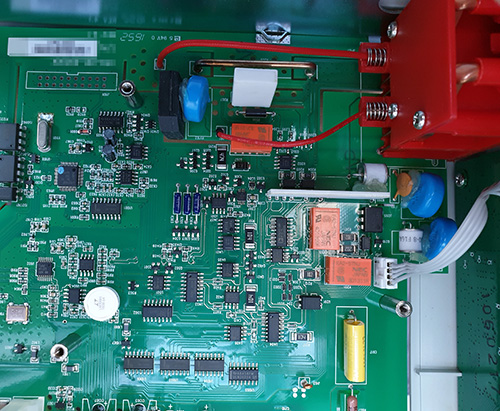
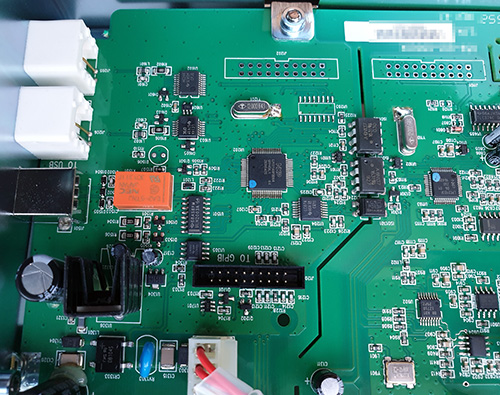
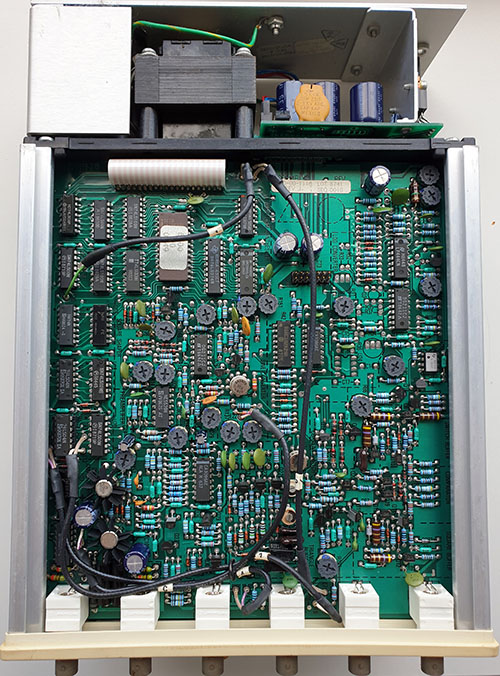
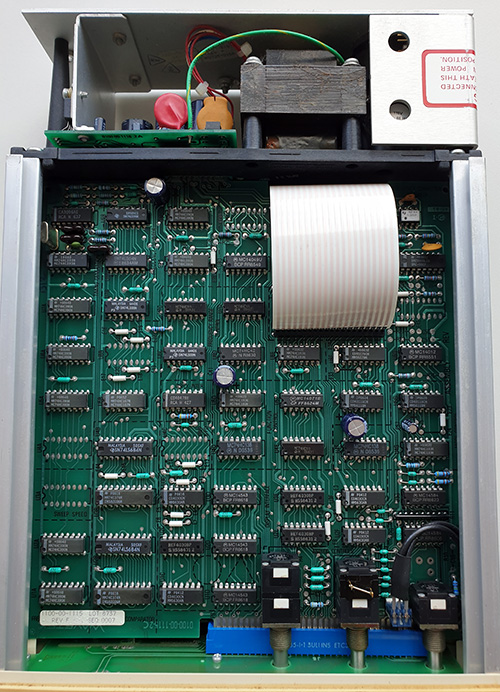
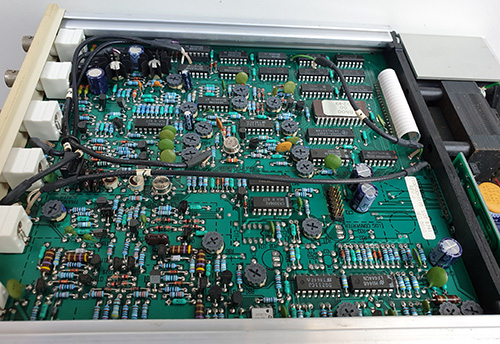
The Ware for November 2022 is shown below.
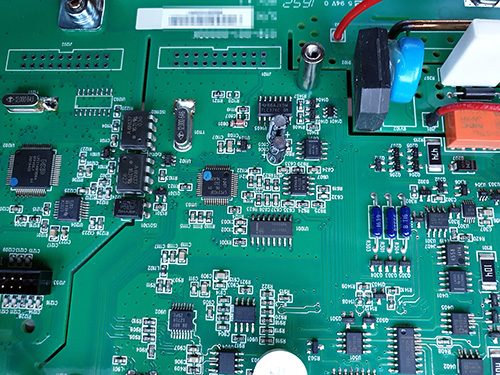
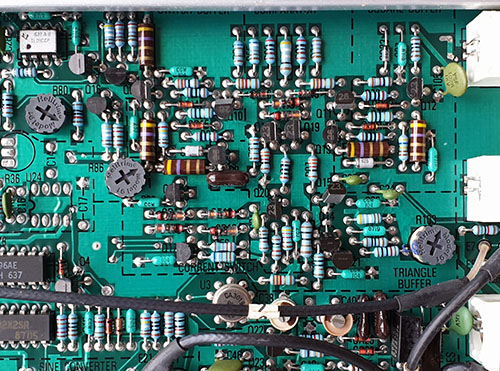
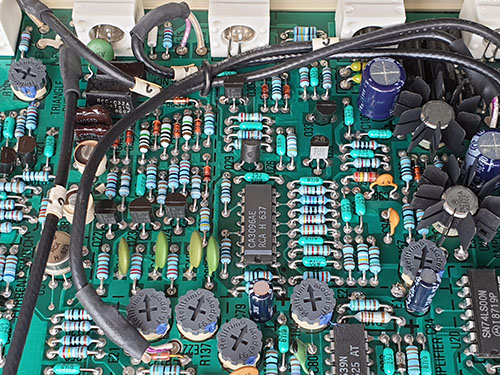
A grounded guard ring is placed around some of the most sensitive analog traces; I would love it if someone could teach me why the soldermask is removed for these guard rings. I imagine there must be some motivation to retain this motif even into mass production, since the mask-less traces run between SMT pins, which I have to imagine incurs a potential yield impact, or at the very least it makes rework more challenging.
Also, yet another tamper-proof seal broken:

It was just a matter of time…such is the fate of any seal within my reach!